The elaboration of glass from primary components is a complex process, the complete description of which requires the use of various characterization techniques. The use of global analysis techniques such as differential thermal analysis and/or thermogravimetric analysis (DTA-TGA) provides thermal information, but does not describe the chemical processes involved. A technique often used is to heat treat samples at different temperatures, soak them at room temperature and then identify the phases formed by XRD, SEM, etc. This technique is time consuming and tedious. It can lead to biases that are linked to the sample quenching (crystallization of molten phases at high temperature, amorphous phases that are not identified by XRD, for example) or to the choice of the quenching temperatures (some rapid transformations or those taking place in a narrow temperature range may not be identified). To overcome these limitations, it may be necessary to implement in-situ characterization techniques, where the analysis is carried out during the heat treatment. In-situ high temperature Environmental Scanning Electron Microscopy, ESEM, possibly coupled with EDS analysis, is a technique that is particularly interesting for identifying the chemical reactions and various transformations taking place between the primary components and then between the different phases formed1.
Two studies using this technique have recently been carried out to investigate high temperature chemical reactivity during glass elaboration. They will be used as examples to illustrate the contribution of this technique in this particular field of study.
The direct observation of the elaboration of a glass melt from simple compounds was conducted from room temperature to 1000°C2 (Fig. 1). This first step allows the determination of the main morphological transformations and the temperatures at which elemental distribution maps should be recorded. Coupling this information allows us to determine that the sequence of chemical reactions is as follows: 1) dehydration and decarbonation of the RT compounds up to 500°C, 2) From 500°C to 550°C, beginning of the reaction of Na-bearing compounds with silica (in particular), and increase of this reaction up to 567°C. Some compounds, such as sulfates or alumina, are not incorporated into the glass at this stage. 3) At 630°C, the compounds MgO, CaO, and partly Al2O3, are dissolved in the glass melt. The solid sulfates float to the surface of the glass bath. 4) At 647°C, the composition of the glass melt is homogeneous and the sulfates form lakes on the surface of the silicate melt. These steps clearly illustrate the phase separation processes described in the literature (arrow in Fig. 1). 5) When the heating is prolonged to 1000°C, the volatilization of sulfates is observed, as well as the (unexpected) precipitation of phases that float on the surface of the glass bath ((Ca, Mg)2SiO4, ZrO2, iron and zinc oxides).
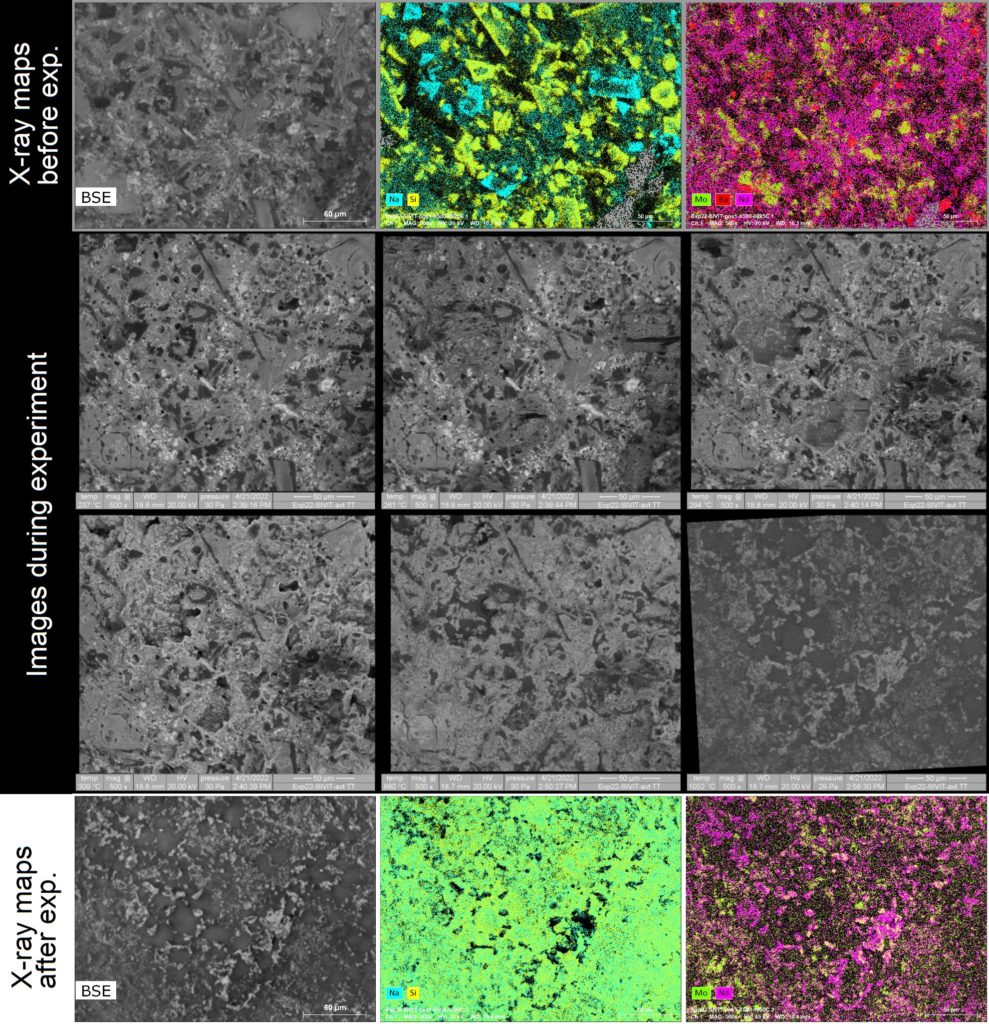
A second series of experiments was carried out to study a new way of vitrifying nuclear wastes which consists of introducing the waste-containing liquid directly into the molten glass, called liquid feeding3. The aim of the study is to identify the reactivity of a glass with chosen compounds (NaNO3, Ba(NO3)2, Mo compounds, REE compounds, etc) and to determine the ability of the glass to incorporate these elements during a heat treatment. The same approach as described above was applied. It was possible to qualify the chemical reactions between the compounds alone, and then their reaction with the glass and the molten glass (Fig. 2). When the different compounds are observed separately from the glass, they do not react chemically together and only physical processes (decomposition and / or melting) are clearly identified. When they are mixed with small pieces of glass (glass frit) prior to the experiment, the global behavior is more complex. Indeed, below the softening temperature of the glass, the compounds react as if there is no glass. At higher temperature, the formation of Na2O yields to the fast reaction with glass. The melting of glass is observed is at temperature lower than 500°C. When temperature reaches 1000°C, crystals containing REE elements and molybdates remain present at the glass melt surface. REE silicates and CaMoO4 crystals are formed during the heat treatment and then not dissolve due to the lower temperature than the nominal glass synthesis (1100°C, 1200°C)
In order to complement the data obtained during in-situ ESEM experiments as described above, complementary experiments can be performed. For example, it is often interesting to stop experiments at a given temperature. Then the analysis of the metallographic preparation of the sample makes it possible to qualify the equilibria between phases and to quantify the compositions of the compounds in equilibrium.
These two examples of in-situ HT-ESEM studies of glass elaboration clearly illustrate that this technique is well adapted to the identification of phase transformations and to the characterization of chemical reactions in complex systems. It allows the identification of reaction pathways and the monitoring of the behavior of particular elements during the heat treatment. The experimental strategy should be adapted precisely to the system to be studied according to the physicochemical properties of the precursors and glasses (liquidus temperature, viscosity). Thus in-situ HT-ESEM can be coupled with other characterization techniques to complete the description of chemical and/or physical reactivity in the system to be studied.