The Hanford Waste Treatment and Immobilization Plant (WTP) at the Hanford Site is currently nearing startup to immobilize waste from plutonium production. For the first 10 years the WTP will be fed directly with a low-activity fraction of waste extracted from the 200,000 cubic meters Tank Farm inventory. The WTP is now commissioning and starting up two electric, Joule-heated melters to vitrify low-activity waste (LAW) into a borosilicate glass matrix. In general, LAW melter feed is composed of the Hanford waste tank supernatant liquid, comprising aqueous solutions of sodium, potassium, aluminum, nitrates, nitrites, sulfates, chlorides, fluorides, and organics (such as acetates, formates, or glycolates), together with glass-forming additives, such as silica sand, kyanite, wollastonite, olivine, or zircon. Furthermore, reductants, such as sucrose, formic acid, urea, starch, cellulose, or glycolic acid, are added to LAW melter feeds to prevent excessive foaming by hastening the denitration, i.e., by evolving gases from nitrates and nitrates at temperatures below the primary foaming onset. Besides diminishing primary foaming, the changes in reaction pathways caused by the presence of reducing agents affect also other conversion processes – for example, the retention of radioactive technetium or the composition of gaseous emissions generated during the vitrification process.
In this contribution, we will address the effect of sucrose, a preferred organic reductant due to its solubility in water and high reducing potential, on the melting process during waste vitrification. Using the combination of feed volume expansion test, thermogravimetric analysis, evolved gas analysis, x-ray diffraction, and scanning electron microscopy with energy dispersive x-ray spectrometry, we experimentally analyzed and theoretically described the changes in the feed conversion reactions, showing that as the fraction of sucrose in the feed increases, the extent of foaming decreases. Interestingly, although moderate levels of sucrose addition reduced foaming, they did not significantly affect the glass production rate. This can be attributed to the observation that it is not the foam volume, or the foam porosity, that controls the heat flow from the melt to the cold cap, but the temperature at which foam collapses. Hence, reducing the extent of foaming without affecting the foam collapse temperature does not noticeably affect the rate of melting. Significant foam suppression and decrease of foam collapse temperature were however observed when the carbon-to-nitrogen ratio in the melter feed exceeded one. While such high sucrose levels would increase the rate of melting, they could also lead to the reduction of transition metal oxides to elements.
Moreover, we varied the sucrose fraction in the feeds while measuring the partial pressure of oxygen, iron speciation, and Re (as a surrogate for Tc) retention in two different low-activity waste melter feeds. The results show that almost no Re is lost during the feed conversion reactions below 800°C. The retention of Re at 1150°C then increases with decreasing partial pressure of oxygen, i.e. with increasing sucrose fraction. By reacting with nitrates and nitrites, a higher sucrose content decreases the content of the perrhenate-containing molten salt phase. It is hypothesized that a lower fraction of the salt phase can increase the extent of Re diffusing into the early glass-forming melt, yielding an increased Re retention. We confirmed that a relatively small increase in sucrose level from the current nominal values may significantly improve Tc retention in the melt without forming metallic iron.
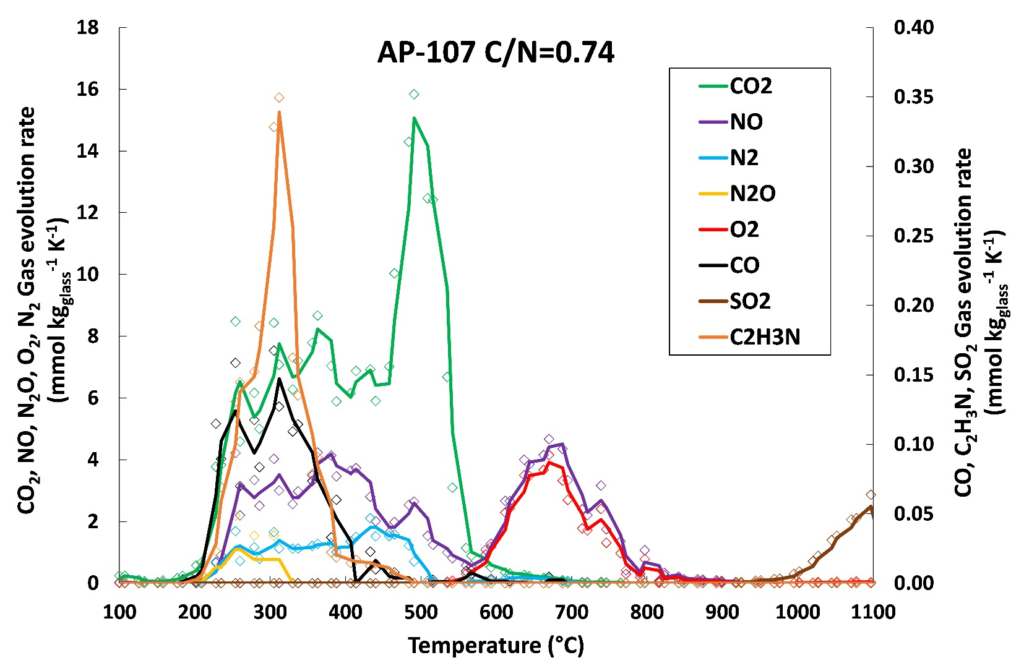
Finally, reaction stoichiometry and linear regression were successfully used to estimate the effect of reducing agents on the composition of gaseous emissions, measured by evolved gas analysis. Although linear regression provided satisfactory results, we show that the gas evolution is inherently nonlinear because it depends not only on the content of nitrogen (nitrates, nitrites, nitrides) and organics in the feed, but especially on their ratios, i.e., on the feed redox state. Attempts to estimate the gas evolution based on the feed reaction stoichiometry were satisfactory when a two-step reaction mechanism was considered, consisting of a reaction between organic compounds and nitrates/nitrites producing CO2, NO, and N2, and then the residual decomposition of nitrates and nitrites above ~550 °C, producing NO and O2. The comparison of EGA results with pilot-scale melter data shows that the off-gas emissions trends are qualitatively similar, but the actual values are affected by the differences in melting conditions, atmospheric conditions, or residence time. If these factors are considered, a multiple regression analysis is expected to closely approximate the off-gas compositions for a broad range of melter operating conditions and waste feed chemistries, thus satisfying the need to develop a predictive off-gas emission model and reducing the amount of required physical scaled melter testing.
Abstract
Effect of reducing agents on the redox conditions during melting, Tc/Re retention, and off-gas evolution
Effect of reducing agents on the redox conditions during melting, Tc/Re retention, and off-gas evolution
Richard Pokorny* 1, Jaroslav Klouzek 1, Miroslava Vernerova 1, Petra Cincibusova 1, Pavel Hrma 2, Albert A. Kruger 3
1 University of Chemistry and Technology Prague, Technicka 5/1905, Prague 6, 166 28, Czechia
2 AttainX, Support Services Contractor to the Office of River Protection, U.S. Department of Energy, Richland, WA, USA
3 U.S. Department of Energy, Office of River Protection, Richland, WA 99354, U.S.A.
2 AttainX, Support Services Contractor to the Office of River Protection, U.S. Department of Energy, Richland, WA, USA
3 U.S. Department of Energy, Office of River Protection, Richland, WA 99354, U.S.A.
- Type: Guest oral presentation
- Related categories: High temperature characterization
