The question of silicate glass chemical durability is at the heart of many industrial and environmental issues, with certain glasses, such as bioglasses, needing to transform rapidly, while others, like nuclear glasses, extremely slowly. Due to the wide diversity of the chemical composition for these types of materials and their metastability – no thermodynamic equilibrium can be reached between glass and solution – the evaluation of chemical durability remains a scientific challenge. In this presentation, we review the current state of knowledge on nuclear glass alteration mechanisms and kinetics.
Thanks to the development of novel techniques and international collaborations, progress has come from both understanding the fundamental processes at molecular level and integrating that knowledge in models. Zooming in at the molecular level reveals extremely complex and dynamic processes. The challenge now is to identify the relevant mechanisms to be implemented in macroscopic models. The overall picture and the applications must always be kept in mind. Experiments in laboratories as well as observations of natural systems have demonstrated that silicate glasses can self-passivate by forming amorphous gels on their surfaces, but the fate of the protected glass strongly depends on the environment. The mechanisms of gel formation are still under debate, although recent results strongly suggest that a continuum exists between the dissolution/reprecipitation model and the leaching/in situ reorganization model.
Mechanistic models have been developed at some specific scales, although holistic models still need further development to link the various scales and perform reliable predictions.
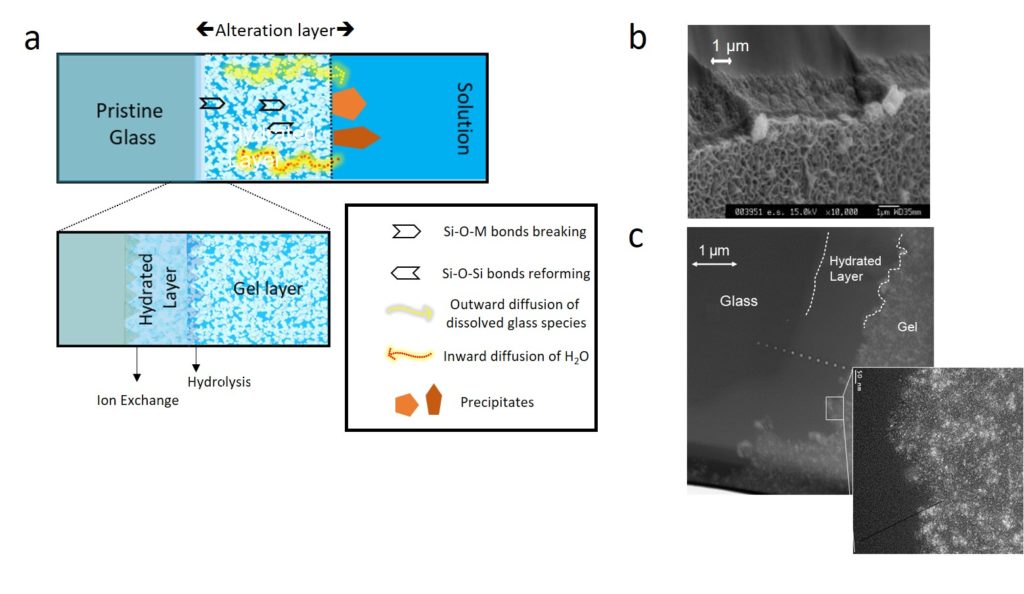
The illustration highlights glass transformation into alteration products. a. Representation of a cross section of an altered piece of glass. The alteration layer is generally made up of a hydrated layer (also called ‘interdiffusion’ or ‘leached’ layer), the gel layer (amorphous hydrated polymerized Si-rich material) which can be made of several sub-layers depending on how it forms, and secondary phases (precipitates). b. Scanning Electron Microscopy image of a piece of SON68 glass, a 30 oxide borosilicate glass, altered for 2 months at 150°C in deionized water. The sample was broken and observed from the edge. Secondary phases precipitated on the top of the gel layer consist of poorly crystallized phyllosilicates. c. Transmission Electron Microscopy bright field image of a SON68 glass sample altered for 26 years at 90°C in granitic water. Pores within the gel can be seen in bright.